This week in AP Bio we studied gene expression and central dogma. We did a bunch of packets and lectures throughout the week to introduce it. First, we looked into central dogma – the flow of genetic information from DNA to proteins, then DNA coding to RNA, then DNA to RNA to proteins. There are two main steps – transcription and translation. First is transcription which is where DNA is being transcribed into mRNA which can be divided up into four stages.
- Initiation
- The DNA molecule unwinds and separates and creates small opening where RNA polymerase binds to the promoter of the template DNA strand. In prokaryotes RNA polymerase binds directly to the promoter and in Eukaryotes RNA polymerase requires an assemblage of transcription factor proteins to be able to bind to the promoter.
- Elongation
- Moves in a 5′ to 3′ direction. RNA polymerase goes along the template strand, creating a mRNA strand containing the important genetic information. The coding strand will have the same sequence with thymines replaced by uracil.
- Termination.
- In prokaryotes there are two ways in which transcription is terminated. Rho-dependent termination- the Rho protein destabilizes the RNA-DNA hydrogen bonding at RNA polymerase and ceases transcription. In Rho-independent termination, the transcript bases hydrogen bond with themselves, fold back and pull the transcript out of the RNA polymerase.
- Post-transcriptional mRNA Processing
- Introns are removed and the exons are spliced together to form a mRNA molecule consisting of a single protein-coding sequence.

Next is translation, which is made up of a similar but different process involving 3 steps; initiation, elongation and termination.
- Initiation
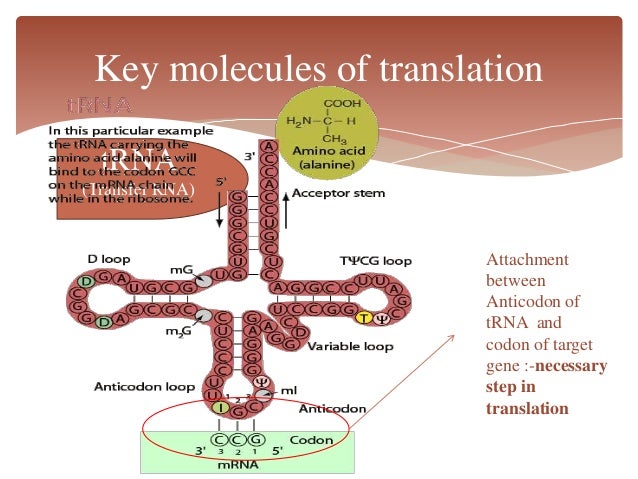
- The mRNA attaches to the small ribosomal subunit o at the 5′ end of the mRNA molecule and moves in a 3′ direction until it meets a start codon (AUG) at the P-site. tRNA binding at the ribosome is mediated by an “anti-codon” loop in the tRNA molecule.
- Elongation
- The ribosome travels down the message, reading codons and bringing in the proper aminoacyl tRNA’s to translate the message to the protein. The new tRNA is brought into the ribosome A site, where it is matched with the codon being presented.
- Termination
- Translation is stopped when a stop codon (UAA, UAG, UGA) is encountered and a release factor binds to the A-site. The polypeptide chain is released and the ribosome disassembles.
Next, we can take look at gene expression. There are two types of gene expression which include inducible (it turns the operon on, starts transcription and translations, caused by a new metabolite which needs enzymes to get metabolized, operates in a catabolic pathway, and the repressor is prevented by the inducer from joining the operator gene) and repressible (it turns the operon off, stops transcription and translation, caused by an excess of existing metabolite, operates in an anabolic pathway, and aporepressor is enabled by a co-repressor to join the operator gene).
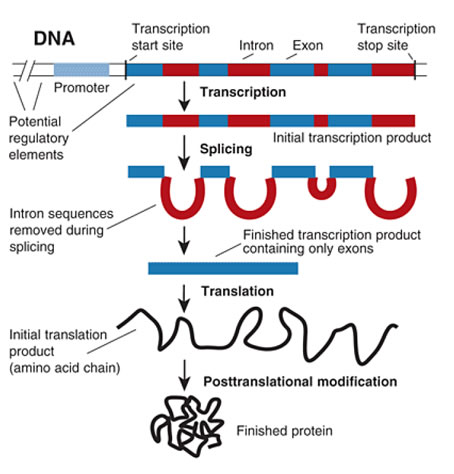
This week was focused on the big idea 2 – understanding within living organisms. I enjoyed doing the packet regarding dog genes this week as it really stimulated my thinking process and made me work for it in an entertaining way vs. the other packets when you have to google to find the answers. Next week I want to look more at diseases in this area, and how translation/transcription can go wrong in organisms.
Websites
- https://www.nature.com/scitable/topicpage/translation-dna-to-mrna-to-protein-393
- http://www.phschool.com/science/biology_place/biocoach/transcription/overview.html
- https://www.khanacademy.org/science/biology/gene-expression-central-dogma/central-dogma-transcription/a/intro-to-gene-expression-central-dogma



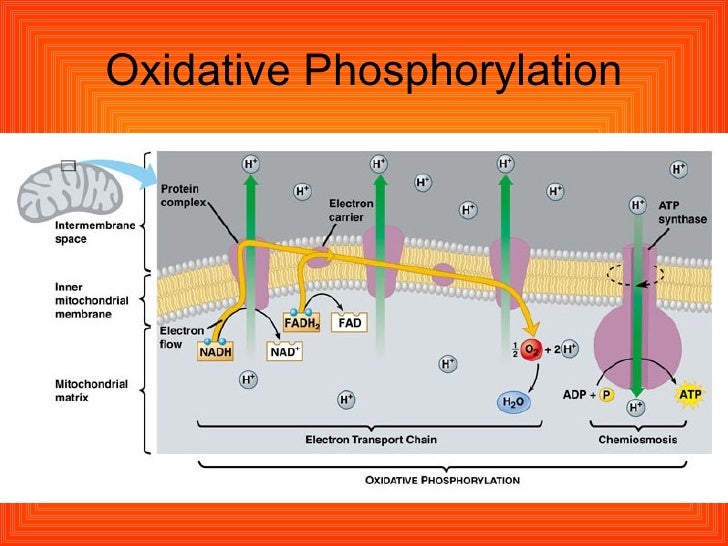
 Anaerobic respiration uses an electron transport chain w a final electron acceptor other than O2 for example sulphate.
Anaerobic respiration uses an electron transport chain w a final electron acceptor other than O2 for example sulphate. 


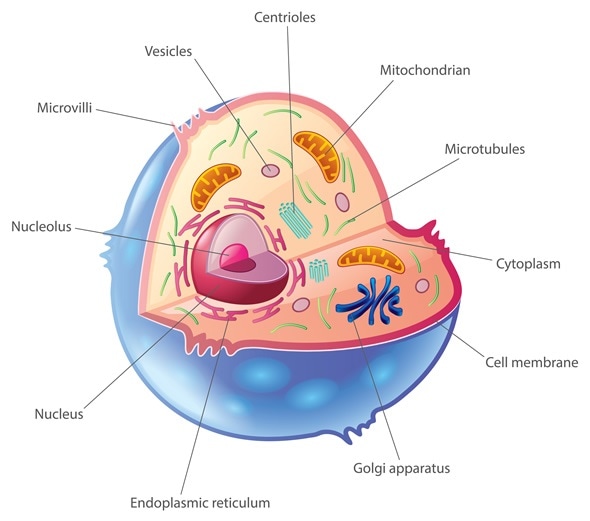
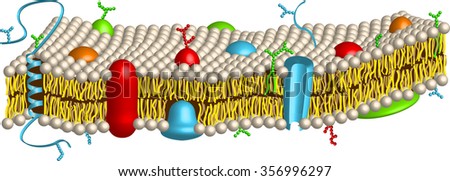
 The cell membrane is made up of a phospholipid bilayer with proteins, otherwise known as the “Fluid mosaic model”. This is like the security gate into the cell, helping with boundary, transportation, and communication. The phospholipid found in the membrane is amphipathic, meaning it is both polar and non polar, and create a semi-permeable membrane. The membrane proteins include both integral and peripheral proteins. Integral penetrate both bilayers, while peripheral proteins don’t penetrate but are hovering on top. These assist in creating polarity. The Cilia and Flagella are motility related extensions of cytoskeletal proteins. The centrosome is the microtubule and is only found in animal cells.
The cell membrane is made up of a phospholipid bilayer with proteins, otherwise known as the “Fluid mosaic model”. This is like the security gate into the cell, helping with boundary, transportation, and communication. The phospholipid found in the membrane is amphipathic, meaning it is both polar and non polar, and create a semi-permeable membrane. The membrane proteins include both integral and peripheral proteins. Integral penetrate both bilayers, while peripheral proteins don’t penetrate but are hovering on top. These assist in creating polarity. The Cilia and Flagella are motility related extensions of cytoskeletal proteins. The centrosome is the microtubule and is only found in animal cells.